Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a rare genetic disorder caused by defective fas-mediated apoptosis. Patients often present in childhood with lymphoproliferation, splenomegaly and multilineage cytopenias (Price et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood 2014). Though mutations in the FAS gene account for the majority of cases, an estimated 20% of patients who have no defined genetic cause are classified as ALPS-U (Shah et al. Autoimmune lymphoproliferative syndrome: an update and review of the literature. Current allergy and asthma reports 2014). Heterozygous STAT3 gain-of-function mutations have been reported to encompass a similar clinical phenotype including expansion of signature cells of ALPS; "double negative" TCR-αβ+T lymphocytes (DNTs) accompanied by solid organ autoimmunity and may represent a proportion of ALPS-U cases (Nabhani et al. STAT3 gain-of-function mutations associated with autoimmune lymphoproliferative syndrome like disease deregulate lymphocyte apoptosis and can be targeted by BH3 mimetic compounds. Clinical Immunology 2017).
We identified 12 patients from 10 families with STAT3 gain of functiongenetic variants (Table 1). Here we describe the clinical spectrum of disease of this cohort seen in our institution during the last 15 years with features suggestive of ALPS-like disease.
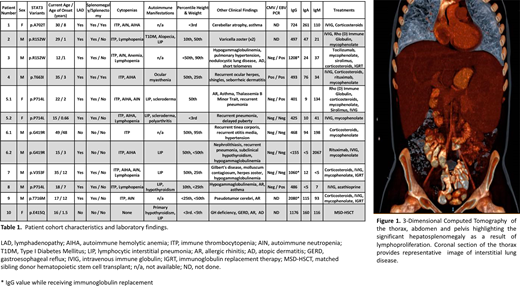
There were 8 males and 4 females. Their median age of symptom onset was 3 years (Range 0.67 - 48 years). The majority of patients demonstrated lymphadenopathy (n=10), splenomegaly (n=9) and multilineage cytopenias (n=10). The immunophenotypic signature of ALPS, DNT cells were expanded in nine patients. A majority of the patients (n=8) demonstrated solid organ autoimmunity with interstitial lung disease being the most prevalent diagnosis (n=7) (Figure 1). As previously described in other cohorts, some patients exhibited growth restriction (n=3) (Khoury et al. Tocilizumab Promotes Regulatory T-cell Alleviation in STAT3 Gain-of-function-associated Multi-organ Autoimmune Syndrome. Clinical Therapeutics 2017; Milner et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 2015).
In contrast to ALPS-FAS where elevated serum vitamin B12 and hypergammaglobulinemia are notable, normal serum B12 and IgG were seen in most patients. Significant hypogammaglobulinemia was seen in three patients with STAT3 GOF defect requiring immunoglobulin replacement therapy (IGRT). Patients experienced both viral (varicella zoster, herpes zoster and mulluscum contangiosum) and sinopulmonary bacterial infections. Four patients demonstrated chronic Epstein-Barr virus (EBV) viremia with only patient #4 exhibiting cytomegalovirus (CMV) viremia.
Treatment was targeted toward management of cytopenias (n=10) and interstitial lung disease (n=7) with several immunomodulatory therapies. Patient #3 received tocilizumab therapy as described in other series (Milner et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 2015). A single patient in this cohort, patient #10 had undergone successful matched sibling hematopoietic stem cell transplant
It is important to consider STAT3 genetic variants in patients with lymphoproliferation, cytopenias and solid organ autoimmunity (Milner et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 2015). Patients with prior diagnosis of ALPS-U should be revaluated for the presence of novel genetic variants as this may guide therapy decisions. At this time, standardized treatment for STAT3 gain-of-function remains to be elucidated with novel targeted therapies currently under investigation.
Rao:novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.